Why it is futile to try and boil your Mash at a specific Temperature
Gert BosmanFirst published on Distillique's website in 2015 by GM Bosman
In the distilling industry, there are many myths which have persisted over many years. This is especially true amongst Home Distillers or Cultural Distillers.
One of these myths (that lead to many an argument during some of our training sessions) is the belief that you should boil your mash at a certain temperature to “boil off” the alcohol before the water starts boiling.
Almost all Home and Cultural Distillers, and especially "Web Educated" Distillers believe this. "You distill at THIS temperature". But nobody can explain why they all have a different temperature!
Now we all know that alcohol boils at 78.3˚C and water at 100˚C (at standard atmospheric pressure of 101.325 kPa) – and this fact has lead to the growth of this distilling myth: “That you should boil your mash at a specific temperature to get the alcohol first".
We have been asked numerous times at which temperature we boil our mashes to get “good alcohol”.
The answer to this is not easy without explaining some physics and thermodynamic principles first.
Raoult was the first to discover in 1882 that different liquids, when mixed, will NOT boil at their individual boiling temperatures, but at a different temperature, depending on the amount of each liquid present in the a solution.
This simply means that, according to Raoult, a 50/50 solution of two liquids, will boil at a temperature that is the average of the two liquids temperature.
I.e. Water boiling at 100 degrees and ethanol at 78 degrees. A solution of 500ml water and 500ml ethanol will therefore boil at 89 degrees - halfway between. Should the concentration of ethanol be greater (lets say 750ml) vs water (250ml), then the boiling temperature of the solution will be closer to the boiling temperature of the greater component, in other words lower. Should the reverse be true, and the concentration of water (at 750ml) is greater than the amount of ethanol (250ml) then the boiling temperature of the solution will be closer to the boiling temperature of water, in other words, higher.
Obviously in a Fermentation we are not just dealing with Ethanol and Water though - we have Methanol, Acetone, Hydrogen Sulfide, Fusil Oils, Acids, Esters, etc. Each of these components, liquids and dissolved solids will have an impact on the boiling temperature of the fermentation. For sake of argument (and to avoid complicating matters) we will however just focus on Ethanol and Water.
Now, this fact of the concentration of different liquids and their individual boiling points impacting the boiling point of a solution is known in physics as “Raoult’s law” and is valid for “ideal solutions”, normally portrayed as a nice straight line on a Graph.
Unfortunately a solution of water and ethanol is however seen as a "non-ideal" solution, and therefore the results are a bit different. Instead of a nice straight line, here we use the proven thermodynamic and physics concept of “phase diagrams”. A phase diagram is a graph indicating at which temperature a solution will start boiling (or condenses) for the various percentages of liquids in the solution.
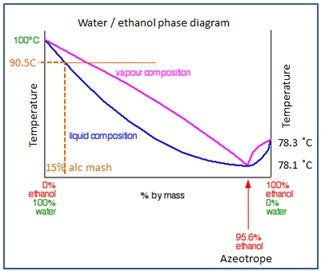
In the phase diagram, everything above the pink line is vapour (or gas), below the blue line everything is liquid, and between the blue and red line, a mixture of gas and vapour exists. The blue line is also called the boiling, or bubble line, while the red line is also called the dew point, or condensation line.
This graph also explains why we cannot distil to purer levels than 95.5% alcohol (using normal distillation methods). This is called the alcohol/water azeotrope.
From the graph, we can see that, for example, a mash with 15% alcohol, will boil at a temperature of 90.5 ˚C. (at standard atmospheric pressure). No matter how big or hot a flame (energy input) we put under our kettle, the mash WILL boil at this temperature and this temperature alone.
As we continue to put heat / energy into the kettle, the temperature of the vapour directly above our mash will not become hotter or colder than the boiling temperature. The energy will however be used to turn more liquid in the boiling vapour, into vapour (the area between the red and blue lines) until all the liquid is vaporised (at the red line).
As we can see from the solid orange line (of the 15% mash example) is that it crosses the red line and that, if we look at the composition of the vapour at that point, the vapour now contains MORE alcohol than the 15% in the mash. In other words, the mash boils at the 90.5C temperature and the resulting vapour contains a richer alcohol content.
If we now condense this alcohol rich vapour, it will condense into a liquid which is higher in alcohol content (the basic mechanism how distillation works).
BUT ... because we extract the alcohol richer vapour, the alcohol percentage in our mash reduces. As the percentage of alcohol in our mash reduces, so the boiling temperature of our mash rises.
The conclusions we can draw from the alcohol/water phase diagram is as follows:
1. The boiling temperature of a mash depends on the percentage of alcohol (and other components) in the mash. We therefore cannot decide at which temperature we want to boil our mash, as we do not know EXACTLY which components formed during fermentation and in which concentrations.
2. As we distil, the percentage of alcohol in our mash slowly reduces, and the boiling temperature slowly rises.
3. If there is no more alcohol left in the mash, the boiling point will reach 100 C – that of the last remaining water in the mash (just before our kettle will boil dry).
Off course the phase diagram can also be used in “reverse”. For example: If we collect our alcohol from a pot still at 55% alcohol, we can use the graph to see how much alcohol is left in the mash. If for example, our distillate reaches 20% alcohol, we would see on the graph that the mash only contains about 2% alcohol.
The above is true ONLY for a proper pot still with no internal reflux.
In a later article we will discuss internal reflux in stills and then understand why different still designs give different results when distilling.
Attend one of our distilling courses and you will learn how the phase diagram is used and how to determine your own still’s natural internal reflux ratio…and off-course how to use this to make really good spirits! ...and why some stills have thermometers in the boiler (kettle) and another one close to the top.
