Can the use of Copper Stills cause Cancer?
adminRecently an Article has been spread around through various Online Forums and Social Media pages, calling into question the use of Copper in Stills.
The basis of this article is that during distillation Copper catalyses substances found in the fermentation into a compound called Ethyl Carbamate, and that this compound is Carcinogenic, and we should therefore not be using Copper Stills or Copper in Stills.
At first, we ignored the articles, for the following obvious reasons:
- People have been using copper stills for close to 2 000 years, and there has never been any direct link between using copper stills and cancer
- This is not the first time the company that wrote the article has used supposed “scholarly” articles to validate their still design choices in order to promote the use of their stills. The previous articles made no sense; hence we were pretty much biased against this article from the get-go.
- A short search shows that Ethyl Carbamate is classified by the IARC (the International Agency for Research on Cancer) as a Class 2A Carcinogen – Possibly Carcinogenic to Humans. Alcohol is classified as Class 1 Carcinogen – Definitely Carcinogenic to Humans (BUT you have to take into consideration the concentrations and exposure times).
FACT: Alcohol on its own, is a much higher risk factor than Ethyl Carbamate. In the quantities responsible drinking is consumed at, Ethyl Carbamate in spirits becomes a non-issue (expect for fear mongering and unscrupulous marketing purposes to promote or justify stills for potable spirits that excludes copper to reduce costs)
So it seems kind of pointless to worry about something that may or may not be in your drink, that may or may not be there in high enough concentrations, to possibly or not contribute to your risk of cancer, while you are consuming something that will contribute to your risk of cancer.
In any case, a lot of our trainees and clients have taken note of this article, some forwarded it to us (laughingly) and others had serious questions. We also noted that many individuals on these groups were actually taking it seriously, and even more used it to open up the old Copper vs Stainless Steel Still argument.
Some comments that drew our attention (paraphrased):
- “Copper in a still is a crutch used by Distillers that cannot make proper fermentations.”
- “The believers in Copper Stills will never listen to science as they cannot back their claims up with science.”
Why do we use Copper in Stills?
If we look at the Timeline of Metals used from Antiquity, we see that Gold was being processed around 6000 BC, Copper 4200 BC, Silver 4000 BC, Lead 3500 BV, the Bronze Age ran from 2300 to 700 BC, Tin 1750 BC, Iron 1500 BC, etc.
The first copper stills were constructed by the Greeks and the Egyptians around 100 AD. Prior to that they had already constructed stills from ceramics and glass as well.
Now the reason for their choice is lost to antiquity, but we can make some educated guesses.
- Copper is a soft, malleable metal that was (and is) relatively easy to shape or form
- Copper is a good conductor of heat
- Copper’s melting point is high enough that they could use it directly in fires without running the risk of the metal melting (unlike lead – thankfully). And before you Google it – Lead Melts at 327.5° Celsius, and Copper at 1 085 °C.
That was it – that was basically (we assume) why we used copper in still construction initially, and it just became standard / common / traditional thereafter to do so, and everybody continued to do it.
When did we start using Stainless Steel for stills?
Stainless Steel was developed in 1913, a 116 years after the discovery of Chromium (1797) which was required to produce this “rustless metal”. Suddenly, we were trying to make everything out of Stainless Steel. The Fashionably Rich hid away their silverware, because now you had to have Stainless Cutlery to prove your wealth and status.
Stainless Steel was suddenly the answer to everything – it was highly corrosion resistant, heat resistant (melting point of 1 200 °C), it was formable, weldable, durable and strong. And, at least later on, it became cheaper than other metals.
Some interesting milestones for Stainless Steel are:
- Between the years 1919 and 1923, the use of stainless steel was adapted to the manufacturing of surgical scalpels, tools, and cutlery in Sheffield.
- In the early 1920s, a variety of chromium and nickel combinations were tested. Stainless steel was referred to as “18/8” to indicate the percentage of chromium and nickel in the steel.
- In 1925, a stainless-steel tank was used to store nitric acid, thereby establishing the fact of this unique metal's resistance to corrosion.
- In 1926, the first surgical implants made of stainless steel were performed.
- The hygienic aspect of the stainless steel was demonstrated in 1928 when the first stainless steel fermenting vessel was used to brew beer. Since then the food and beverage industry have widely used this metal.
- In the 1930s, the first stainless steel train was built in the USA.
- The year 1931 witnessed the creation of the first stainless steel aircraft.
- By 1935, stainless steel kitchen sinks were widely used.
- Type 430 stainless steel (ferritic chromium alloy) was used to make a wire 0.1mm in diameter for a voice-recording machine.
- In 1954, the first stainless steel underwater TV camera was manufactured.
- In 1966, the first tidal power station with stainless steel turbine blades was completed in France.
- In the 1980s, stainless steel was used to build the longest movable flood barrier in the world on the river Thames.
- Global production of stainless steel reached 31 million Mt in 2010.
- About 11 million washing machines with stainless steel drums were produced in China in 2010.
In 1936, Ford Motor Corporation even built a Stainless-Steel Ford Tudor (which I personally found both fascinating and beautiful - hence the picture).

Figure 1
But back to Stills …
We could not find any record or indication of when the first Stainless Steel Still was constructed, but as the first recorded use (according to the timeline above) in the beverage industry was in 1928, we can safely assume it was after that.
The reasons were that stainless was cheaper and more durable than copper, and also easy to clean.
The problem they would have found though, is that when you use a pure Stainless Steel Still, your product tastes horrible when it comes out the other side. Specifically, sulphurous. Why?
Why do we need Copper in Stills?
The quick and dirty, bullet point answer is:
- Yeasts release sulphur when they’re doing their business.
- In a non-copper still, that sulphur stays with the liquid.
- With copper, the sulphur molecules bind with the copper, making hydrogen-sulphide, which becomes copper sulphate.
- The copper sulphate remains in the still rather than going out with the liquid and is then cleaned out when the still is cleaned.
- Removing the sulphur compounds (which taste bad in any case) allows the fruity-smelling esters (organic compounds that can give off recognizable aromas) to shine through in the finished spirit. The more interaction with copper, the more sulphur compounds are removed.
The more scientific explanation, which most of you will either skip or speed-read through, is as follows:
A lot of what we know today about distillation comes from knowledge obtained through years of trial and error – none more so than the reason for copper in stills. Using copper stills as we always had, we never realized or considered that it played a role in quality. This ONLY became a consideration after the first trails in stainless steel stills.
Now that the question was raised, the role of copper was studied, and we find out that through the centuries copper has acted as a ‘silent contributor’ to spirit quality.
The availability of clean copper inside the still being vital to allow complex chemical reactions to take place, removing highly volatile sulphur compounds – chief among them dimethyl trisulphide or DMTS. The aroma of DMTS has been described as rotten vegetables – not really what one would call a desirable compound in a distilled spirit.
Some schools of thought also states that copper helps in the formation of esters, which tend to give the spirit a fruity character – others feel that the removal of sulphides merely allow the esters from the fermentation to present themselves. Whichever story you prefer, the end result remains the same.
In controlled testing – published in the Journal of the Institute of Brewing, 117(1), 106-112, 2011 by Barry Harrison, Olivier Fagnen, Frances Jack and James Brosnan – based on running a pure stainless steel still and a pure copper still of the same design, the following results were discovered:
Sensory Analysis. Sensory profiles of the new make spirits produced using the full copper and full stainless-steel stills are shown in Fig. 2.
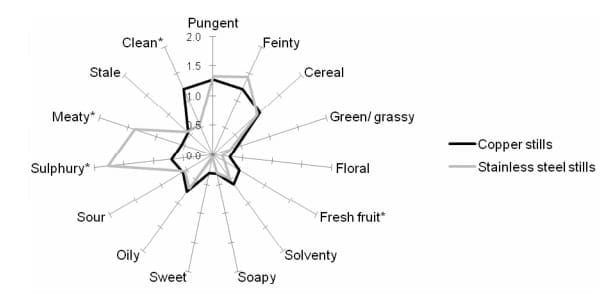
This analysis showed the impact of using stainless steel stills in place of copper ones. The sensory attributes which most characterised the copper stills new make spirit were cereal, feinty, pungent and clean. Using stainless steel resulted in the new make spirit being described as significantly less clean. This was attributed to, as might be expected, a significant increase in the levels of sulphury and meaty aromas when using stainless steel.
Sulphur Compounds. The new make spirits produced using the full copper and stainless-steel stills were compared in terms of sulphur compound levels (Fig. 3). Figure 3A shows the levels of known sulphur compounds. Of these, only DMTS showed a statistically significant difference between the copper and stainless-steel stills. This compound was present at significantly higher levels in new make spirit produced using stainless steel stills.
When the aroma detection threshold is considered (33 ppt in 20% ethanol), DMTS was detected at levels likely to have a direct impact on new make spirit aroma. This suggested that DMTS was a contributor to the elevated levels of sulphury and meaty aromas in the stainless-steel stills new make spirit.
Several unknown sulphur compounds were also detected in the new make spirits produced. The levels of the four most quantitatively important of these are shown in Fig. 3B.
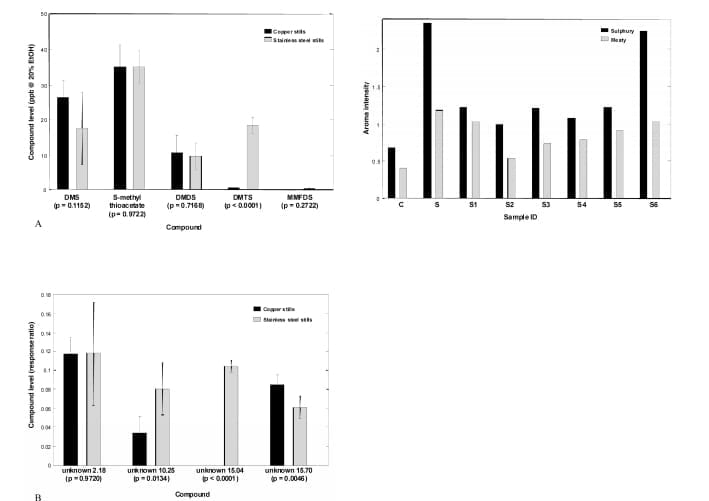
It was found that three of these unknown compounds were present at statistically significantly different levels in the new make spirits produced from the copper and stainless-steel stills. Unknown 15.70 was found at a higher level in the copper stills new make spirit whilst unknown 10.25 and unknown 15.04 were found at higher levels in the stainless-steel stills new make spirit. Unknown 10.25 and unknown 15.04 were therefore more likely to have made a contribution to the sulphury and meaty aromas which characterised the stainless-steel stills new make spirit.
Without going into too much detail here (for those that like detail, you can read the full research paper on this link), the experiment also tested the impact of placing copper inside a stainless steel still.
The impact of the presence of any copper during distillation was to reduce the DMTS levels in new make spirit. However, it was found that the positioning of the copper in the stills had a large effect on the ability of the copper to reduce the level of DMTS in the new make spirit and placing copper in any single section was unable to replicate the effect of the full copper stills.
There was found to be opposing trends in the wash and spirit stills.
(For clarification – a wash still will be the equivalent of a stripping still, used to produce low wines)
In the wash still, the pot (boiler) was the least effective at reducing DMTS whilst the condenser was most effective. In the spirit still, the pot (boiler) was most effective and the condenser was least effective. The most effective sections for reducing the DMTS level were therefore the wash still (stripping still) condenser and the spirit still pot (boiler).
Now this is followed by a whole discussion on why these areas are better at sulphide removal, and the possibility that is has to do with copper corrosion and acidification. Personally, I do agree with their theories, based on personal experience using copper plates that were not properly passivated after acid cleaning – they tend to bind a lot more sulphides on them faster than other plates.
One thing however must be highlighted.
The stills used in these experiments were of the standard Whisky Still design and were not Plate Column or Fractionating Reflux Column Stills. In these still designs we have the capability to increase copper contact in the vapour path through the addition of copper plates, copper mesh packing, copper saddle packing, etc. etc.
This GREATLY increases the copper surface area within a given space, and thereby increases the effective extraction of sulphur compounds within these sections, which can compensate for not having a copper boiler or condenser.
There is enough scientific evidence for Copper Lovers to back their claims on the beneficial sensory effect of copper.
Are there always Sulphur Compounds in Fermentations?
Sulphur is important for the growth of all microorganisms due to the formation of sulphur containing amino acids. Among the ethanol related microorganisms, extensive data has been accumulated for yeast for the species Saccharomyces Cerevisiae – the predominant yeast family used in the fermentation of beer, wine and for distilled spirits.
Yeast has the ability to use various sulphur compounds in contrast to many other microorganisms due to the sulphur pathway in yeast, which allows it to use various organic and inorganic sulphur compounds as sole sulphur source.
The element sulphur can occur in a variety of stable compounds in which it can range from −2 in its most reduced form (sulphide) to +6 in its most oxidised form (sulphate). For all microorganisms, the biosynthesis of sulphur amino acids requires the ability to accumulate sulphur atoms from the growth medium and then the transformation of the transported intermediate compounds into the reduced form of the sulphur atom (S2−).
So, what the hell does all of that mean?
The figure below represents the Sulphur Pathways of Saccharomyces Cerevisiae, as published in FEMS Yeast Research, Volume 17, Issue 6, September 2017. You can read the whole article at this link.
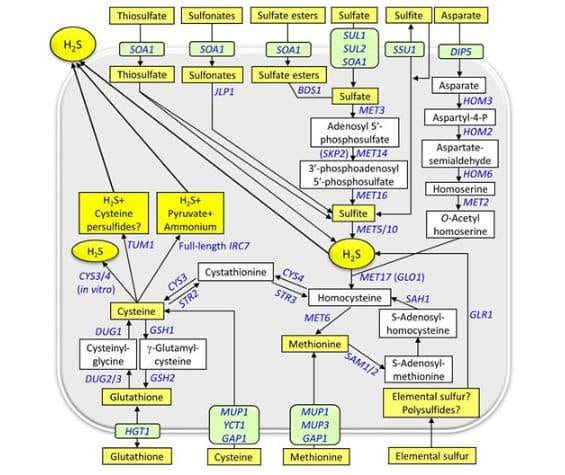
The important piece of information to take from this is that during fermentation, the Sulphur Pathways followed by the yeast produces Hydrogen Sulphide (H2S) from:
- Glutathione (naturally present in plants and sometimes added as part of nutrient mixes)
- Cysteine (present in plants – especially plants that contain proteins like potatoes barley, wheat, rye, spelt, millet, wheat, oats and rice)
- Elemental Sulphur (sprays, pesticides, preservatives, etc.)
- And multiple other sources
Also just note that although this research refers to Saccharomyces Cerevisiae specifically, the same rules apply to all Microbial Strains, just at different levels.
So, the presence of Sulphides in a Fermentation is unavoidable, has nothing to do with your skill at fermentation, and will not be affected by your choice of raw material.
Only the concentrations and amounts are affected by the aforementioned variables.
Is it true that Copper Stills can lead to Ethyl Carbamate which causes Cancer?
Yes and no.
The following is an extract from a research paper published in the Journal of the Institute of Brewing, March – April Edition, 1989, Volume 95, Page 115-119. You can read it at this link.
The formation and distribution of ethyl carbamate (urethane) during pot still distillation was investigated. Formation only occurred if the distillation was carried out in the presence of copper. Removal or chelation of dissolved or suspended copper prevented ethyl carbamate formation. When copper was present, during and subsequent to distillation, formation of ethyl carbamate was time dependent. The degree of formation was maximised between pH 4 and 6. During the second or low wines distillation only 1-2 per cent of the total available ethyl carbamate was collected with the potable spirit fraction. The remainder was distributed between the feints (15 per cent) and the spent less (84 per cent).
The widespread presence of ethyl carbamate in alcoholic beverages was discovered during the mid-1980s.
Studies have shown that most, if not all, yeast-fermented alcoholic beverages contain traces of ethyl carbamate (15 ppb to 12 ppm).
Other foods and beverages prepared by means of fermentation also contain ethyl carbamate.
For example:
- bread has been found to contain 2 ppb
- as much as 20 ppb has been found in some samples of soy sauce
Amounts of both ethyl carbamate and methyl carbamate have also been found in wines, sake, beer, brandy, whiskey and other fermented alcoholic beverages.
It has been shown that ethyl carbamate forms from the reaction of ethanol with urea:
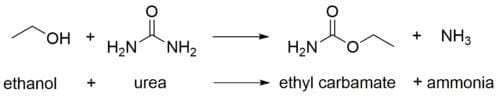
The urea waste product is initially metabolised inside the yeast cell until it builds up to a certain level. At that point, it is excreted externally where it is able to react with the alcohol to create ethyl carbamate.
At some stage Urea was also used as a nutrient in fermentations (it has since been replaced by di-ammonium phosphate or other nitrogen carriers) but has been banned for use in any food and beverage products.
This reaction occurs much faster at higher temperatures, and therefore higher concentrations of ethyl carbamate are found in beverages that are heated during processing, such as brandy, whiskey, and other distilled beverages. Additionally, heating after bottling either during shipping or in preparation will cause ethyl carbamate levels to rise further.
In 1988, wine and other alcoholic beverage manufacturers in the United States agreed to control the level of ethyl carbamate in wine to less than 15 ppb (parts per billion), and in stronger alcoholic drinks to less than 125 ppb.
Furthermore, some strains of yeast have been developed to help reduce ethyl carbamate during commercial production of alcoholic beverages.
Another important mechanism for ethyl carbamate formation in alcoholic beverages is the reaction from cyanide as precursor, which causes comparably high levels in spirits derived from cyanogenic plants, such as rhum agricole. This is where the copper may act as a catalyst.
Is Ethyl Carbamate dangerous?
Ethyl carbamate is not acutely toxic to humans, as reflected by its use as a medicine.
When ethyl carbamate was used medicinally, about 50% of the patients exhibited nausea and vomiting, and long-time use led to gastroenteric haemorrhages, but it must be noted that this is at high concentrations. This medical use was at diluted levels of 7 to 15% in 2 ml ampules – used as an Analgesic (pain medication) and to treat Multiple Myeloma (Cancer of the Blood Plasma).
The IARC (the International Agency for Research on Cancer) has stated that ethyl carbamate can be "reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity in experimental animals".
It must be noted that considering the dosages used in medical treatments were the same as (and higher) than the dosages administered to animals during testing, there was never any direct link proven to the use of Ethyl Carbamate and increased cases of cancer. In the sake of full disclosure though – it does not seem as if there was direct research done on the topic.
It also seems that Asian populations are the highest risk group. Studies in Korea and Hong Kong outlined the extent of the accumulative exposure to ethyl carbamate in daily life among the local population. Fermented foods such as soy sauce, kimchi, soybean paste, breads, rolls, buns, crackers and bean curd, along with wine, sake and plum wine, were found to be the foods with the highest ethyl carbamate levels in traditional Asian diets, and get consumed not only regularly, but daily.
Even there, however, no direct link between Ethyl Carbamate in spirits and cancer could be proven.
So, is the use of a Copper Still dangerous or not?
If we look at the final findings of the previously mentioned experiments, we find the following conclusion:
Ethyl carbamate, once formed, does not readily distil when the alcohol concentration is high. This means that only small proportion, approximately 1-2 per cent of the total available ethyl carbamate, will distil over during collection of potable spirit. As the alcohol strength drops from 64 per cent v/v to about 1.5 per cent v/v, further 15 per cent of the available ethyl carbamate will be collected with the feints. Clearly, the elimination of 84 per cent of the total available ethyl carbamate with the spent lees prevents substantial accumulation in the feints.
If you read the article, you will see that in the stripping run, the Ethyl Carbamate level in the low wines was 18 ppb (parts per billion) immediately after distillation, increasing to 75 ppb 8 hours, as the compounds formed over time. The highest levels, 162 ppb was observed 3 days after the stripping run. Ethyl Carbamate formation also increases at higher temperatures (50 degrees Celsius in the tests – note that this merely speeds it up and does not necessarily increase the total amount formed. Also note that the experiments cited several different levels in different tests – I chose the highest, i.e. the worst-case scenarios.
So, based on this research, and based on the worst case scenario figures, we can therefore calculate that if I distilled my fermentation through copper, and I left that low wines for three days, and redistilled it with a 162 ppb of Ethyl Carbamate, my hearts cut will only contain 2% of that 162 ppb.
The most concentrated hearts cut will be a vodka or neutral spirit run, as that would be kept as close to 96% ABV as possible. So, assuming a worst-case scenario again, of a 10% ABV fermentation strength, a 95% recovery rate of the available alcohol, and a 95% average distillate strength, my Ethyl Carbamate concentration would be in the region of 32.4 ppb. That is a quarter of the allowed maximum of 125 ppb allowed in the USA. (Please feel free to check my math on this and let me know if it’s wrong).
PLEASE NOTE: 125 ppb is the USA limit in spirits. Canada uses 150 ppb to 400 ppb (the latter only in liqueurs and fruit brandies). Switzerland uses 400 ppb.
Long Story Short
- Ethyl Carbamate is potentially dangerous, but we don’t know, so we are careful
- There are limits in place for this reason, but normal distillation processes should be sufficient to reduce the concentration below the recommended limits
There are other measures you can take to reduce the concentration levels (but I am willing to say they are not required):
- Do not add urea to your fermentation to speed it up. Rather use DAP (di-ammonium phosphate)
- Use proper commercially available wine and distillers yeast strains which has been developed for the production of human consumables rather than for industrial use.
- Rather distill twice (stripping run, followed by spirit run)
- Store your low wines (specifically) and final spirits in cold(er) environments
- Treatment of low wines with EDTA greatly reduces Ethyl Carbamate levels
* EDTA is the acronym for Ethylenediaminetetraacetic acid (say that three times fast … or only once)
EDTA is a chemical that binds and holds on to (chelates) minerals and metals such as chromium, iron, lead, mercury, copper, aluminum, nickel, zinc, calcium, cobalt, manganese, and magnesium.
In Conclusion ...
-
Copper is important (which does not mean the entire still needs to be built out of copper - as long as you have copper in the vapour path your are fine) for flavor - And yes we can back that up with science
- No empirical evidence exist or has been published, that linked any deaths to the existence of ethyl carbamate in spirits.
